Abstract
Introduction: CD22 is expressed in >90% of cases of B-cell acute lymphoblastic leukemia (ALL) making it an attractive target for the treatment of B-cell malignancies. Inotuzumab ozogamicin (InO), an anti-CD22 antibody conjugated to calicheamicin, has demonstrated significantly greater efficacy vs standard care (SC) therapies in patients with relapsed/refractory (R/R) ALL in the INO-VATE trial (Kantarjian et al, 2016). Herein, the efficacy and safety of InO vs SC in patients with baseline leukemic blast CD22 positivity ≥90% vs <90% are assessed.
Methods: In this phase 3 trial (NCT01564784) completed on LSLV 04Jan2017, patients with R/R ALL (including ~15% in each arm with Philadelphia-positive ALL) due to receive salvage 1 or 2 therapy were randomized to InO (1.8 mg/m2/cycle [0.8 mg/m2 on day 1; 0.5 mg/m2 on days 8 and 15 of a 21-28 day cycle for ≤6 cycles]) or SC (investigator choice of fludarabine/cytarabine [Ara-C]/granulocyte colony-stimulating factor [FLAG], Ara-C + mitoxantrone, or high-dose Ara-C). Efficacy was assessed in the intent-to-treat (ITT) population; safety was assessed in all patients who received ≥1 dose of study drug. Central flow cytometry was used to assess minimal residual disease (MRD) negativity (<0.01%) and CD22 positivity. The primary overall survival (OS) analysis occurred after 252 events (122 with InO and 130 with SC) were observed on March 8, 2016; data as of this date are presented. All P values are 1 sided.
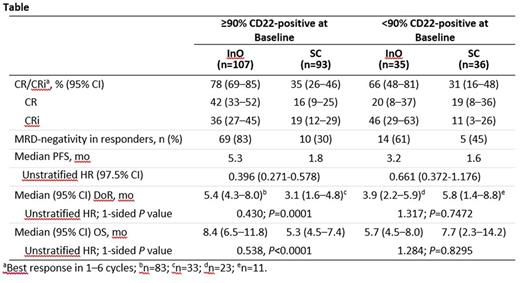
Results: 326 patients (InO, n=164; SC, n=162) were included in the ITT analysis population with both arms being well balanced for baseline characteristics. Most patients in both arms had high (≥90%) CD22 positivity at baseline (InO, 65%; SC, 57%), with only a small fraction exhibiting CD22 positivity <70% (InO, 3%; SC, 11 %). The rate of complete remission (CR)/CR with incomplete hematologic recovery (CRi) was significantly higher with InO vs SC both in patients with higher and lower CD22 positivity (≥90%: 78% vs 36%, rate difference=42, P <0.0001; <90%: 66% vs 31%, rate difference=35, P=0.0015); MRD-negativity rates among responders were also higher with InO vs SC (≥90%: 83% vs 30%, P <0.0001; <90%: 61% vs 45%, P= 0.1985). In the small subgroup of patients with CD22 positivity <70% who received InO (n=5), 3 patients achieved CR/CRi, including 2 patients who achieved MRD-negative status. Progression-free survival was significantly longer with InO vs SC in patients with CD22 positivity ≥90% (hazard ratio [HR]=0.396, 97.5% CI: 0.271-0.578) and showed a similar, albeit non-significant trend in patients with CD22 positivity <90% (HR 0.661, 97.5% CI: 0.372-1.176). Duration of remission (DoR) was significantly longer with InO vs SC in patients with CD22 positivity ≥90% who achieved CR/CRi (HR=0.430, P=0.0001; median 5.4 vs 3.1 mo) but no significant difference in DoR was observed in patients with CD22 positivity <90% who achieved CR/CRi (HR=1.317, P=0.7472; median 3.9 vs 5.8 mo). OS was significantly longer with InO vs SC in patients with CD22 positivity ≥90% (HR=0.538, P <0.0001; median 8.4 vs 5.3 mo); no significant difference in OS were observed in patients with CD22 positivity <90% (HR=1.284, P=0.8295; median 5.7 vs 7.7 mo). In the safety population, cytopenias were the most common grade ≥3 adverse events (AEs) with InO, with similar rates in patients with CD22 positivity ≥90% and <90% (neutropenia, 45.8% and 51.4%; thrombocytopenia, 32.7% and 62.9%; febrile neutropenia, 27.1% and 31.4%); grade ≥3 hepatobiliary AE rates were also similar between patients with CD22 positivity ≥90% and <90% treated with InO (hyperbilirubinemia, 5.6% and 2.9% and veno-occlusive liver disease [VOD; All grade ≥3 VOD events within 2 years of randomization date regardless of causal attribution to study therapy were included], 14.0% and 5.7%).
Conclusion: At least 90% of blasts were CD22-positive in the majority of patients. The greatest benefit from treatment with InO was observed in those patients with CD22 positivity ≥90%, while statistical differences in PFS, DOR, and OS could not be shown in patients with CD22 positivity <90% on ALL blasts. Overall, InO demonstrated a favorable benefit/risk profile for patients with relapsed or refractory B cell precursor ALL independent of CD22 positivity.
Kantarjian: Bristol-Meyers Squibb: Research Funding; ARIAD: Research Funding; Amgen: Research Funding; Novartis: Research Funding; Delta-Fly Pharma: Research Funding; Pfizer: Research Funding. Stock: Pfizer: Consultancy, Membership on an entity's Board of Directors or advisory committees; Seattle Genetics: Consultancy, Membership on an entity's Board of Directors or advisory committees; Amgen: Consultancy. DeAngelo: Glycomimetics: Research Funding; Amgen: Consultancy, Research Funding; Immunogen: Honoraria, Research Funding; Incyte: Consultancy, Honoraria; Blueprint Medicines: Honoraria, Research Funding; Takeda Pharmaceuticals U.S.A., Inc.: Honoraria; Shire: Honoraria; Pfizer Inc.: Consultancy, Honoraria, Research Funding; Novartis Pharmaceuticals Corporation: Consultancy, Honoraria, Research Funding; ARIAD: Consultancy, Research Funding; BMS: Consultancy; Celgene: Research Funding. Jabbour: Bristol-Myers Squibb: Consultancy. O'Brien: Gilead Sciences, Inc.: Consultancy, Other: Research Support: Honorarium, Research Funding; CLL Global Research Foundation: Membership on an entity's Board of Directors or advisory committees; Pharmacyclics: Consultancy, Other: Research Support: Honorarium, Research Funding; Pfizer: Consultancy, Research Funding; ProNAI: Other: Research Support: Honorarium, Research Funding; Astellas: Consultancy; TG Therapeutics: Consultancy, Other: Research Support: Honorarium, Research Funding; GSK: Consultancy; Celgene: Consultancy; Acerta: Other: Research Support: Honorarium, Research Funding; Regeneron: Other: Research Support: Honorarium, Research Funding; AbbVie: Consultancy; Amgen: Consultancy; Sunesis: Consultancy; Aptose Biosciences, Inc.: Consultancy; Janssen: Consultancy; Vaniam Group LLC: Consultancy; Alexion: Consultancy. Wang: Pfizer: Employment, Equity Ownership. Paccagnella: Pfizer: Employment. Nguyen: Navigate BioPharma Services, INC: Employment. Sleight: Pfizer: Employment, Equity Ownership. Vandendries: Pfizer: Employment, Equity Ownership. Laird: Pfizer: Employment. Advani: Takeda/ Millenium: Research Funding; Pfizer: Consultancy.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal